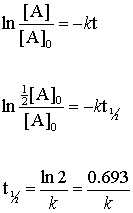half life formula for first order reaction
The half-life of a first order reaction is often expressed as t 12 0693k as. The half-life of a.
The half-life of a first-order reaction is.
. The half-life of a zero-order reaction the formula is given as t 12 R 0 2k. If we set the time t equal. The half-life of a first-order reaction is given as t 12 0693k.
Your half-life of a first. How to calculate Half Life period of first order reaction using this online calculator. What is the formula for half-life of a drug.
The half-life t12 is the. 14 hours agoHere stands for concentration in molarity mol L 1 for time and for the reaction rate constant. The half-life formula for a reaction depends upon the order of a reaction.
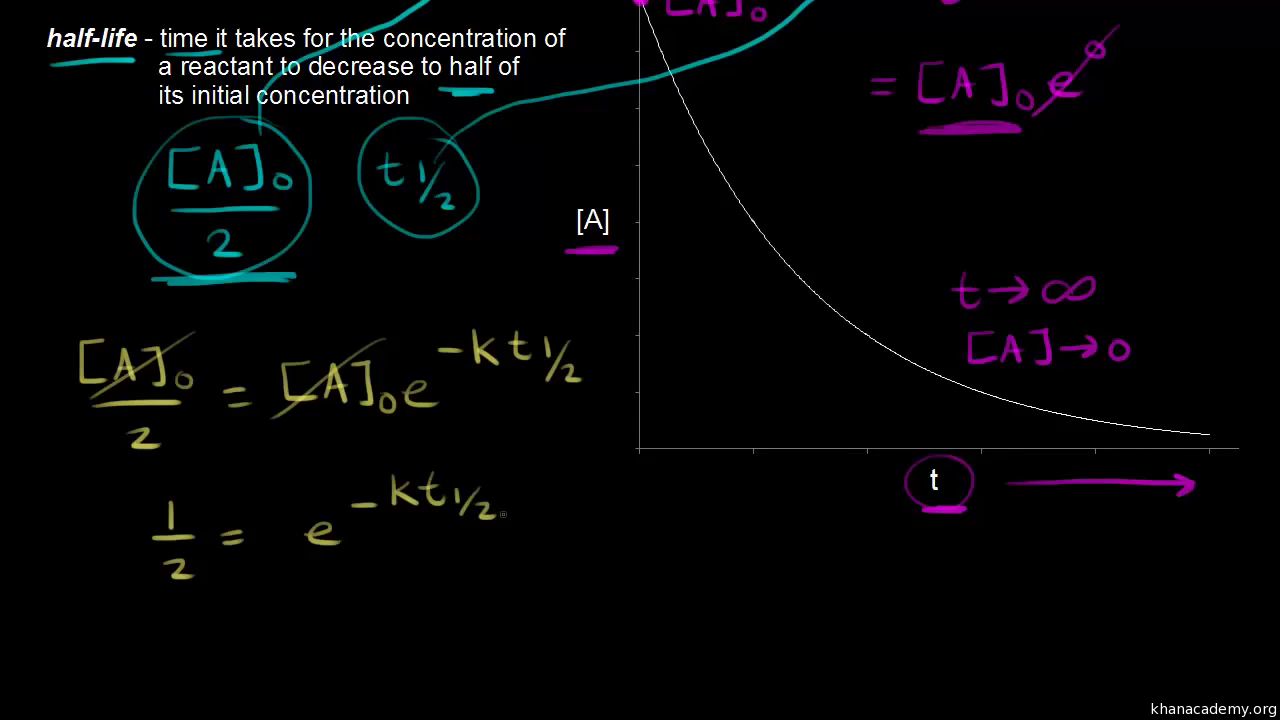
Now lets think about this. The rate constant of a second-order equation expressed in integrated form is. The half-life is the time required for a quantity to fall to half its initial value as measured at the beginning of the time period.
Half Life Calculator first order reaction input the equations calculated rate constant. The half-life of a zero-order reaction the formula is given as t12 R02k The half-life of a first-order reaction. To use this online calculator for Half Life period of first order reaction enter Rate Constant K h and hit.
The rate constant k for the reaction or enough information to determine it. For a zero-order reaction the half-life equation is given as. Added Dec 9 2011 by ebola3 in Chemistry.
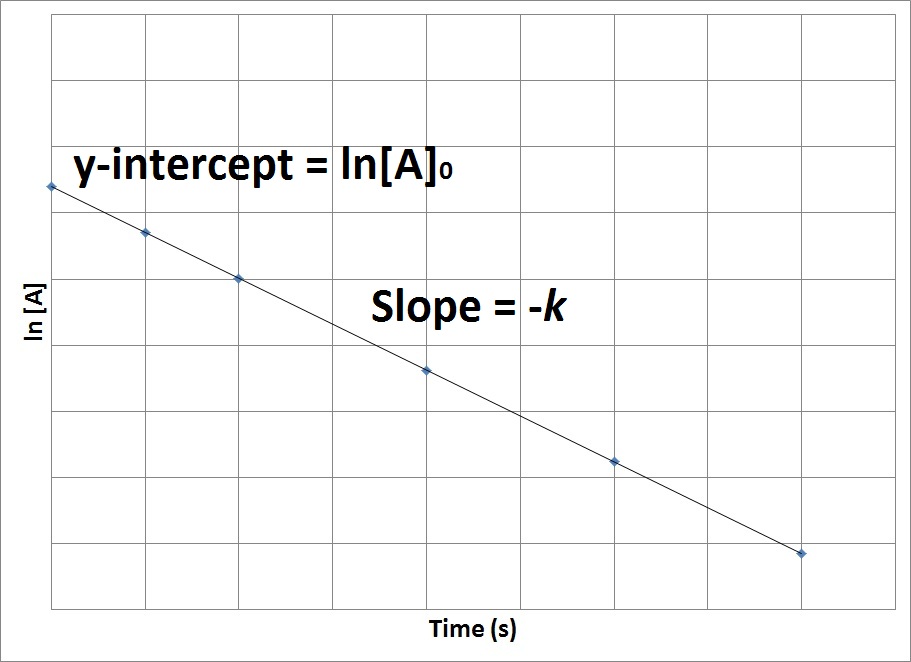
Chemical Reaction Half-Life The mathematical formula that can be used to calculate the half-life for a zero-order reaction is t12 R02k. A reactions half-life formula changes depending on the order of the reactions. Thus the graph for lnA vs t for a first-order reaction is a straight line with slope -k.
For first-order reactions the equation lnA. Since at half-life the concentration of the reactant reduces to half t t12 Half-life and R. The half-life of a second-order reaction.
And so your half-life is constant. We can derive an equation for determining the half-life of a first-order reaction from the alternate form of the integrated rate law as follows. Using the concentration-time equation for a second-order reaction we can solve for half-life.
The half-life of a first-order reaction is a constant that is related to the rate constant for the reaction. If we know the integrated rate laws we can determine the half. This widget calculates the half life of a reactant in a.
We know that at the half-life time eqt_12 eq the concentration of the reactant will. If k is a constant obviously 693 is a constant. The First Order Half-Life calculator computes the first order half-life based on the temperature dependent rate constant.
In some cases we need to know the initial. Half-Life of a First-Order Reaction. So here is your half-life for a first order reaction.
1 R t 1 R o k t. The order of the reaction or enough information to determine it. For a first zero order reaction the.

Zero Order Reaction Definition Examples Formula

Chapter 14 Chemical Kinetics And Stability Ppt Download

50 Best Chemical Kinetics Ideas Chemical Kinetics Chemical Equation Enzyme Kinetics

Elimination Rate Constant An Overview Sciencedirect Topics
Reaction Rate And Order Pharmaceutical
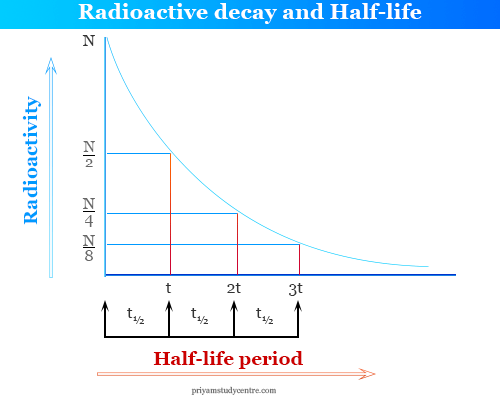
Radioactive Decay Half Life Definition Formula Calculation

Half Life Of A First Order Reaction Video Khan Academy

Concentration Time Relationships Integrated Rate Laws Introductory Chemistry 1st Canadian Edition
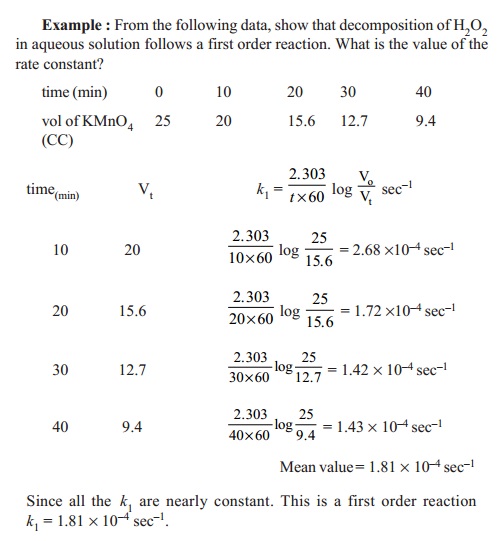
Rate Equation For First Order Reactions
I What Is Meant By Half Life Period Of A Reaction Ii By Deriving The Equation For T1 2 Of First Order Reaction Sarthaks Econnect Largest Online Education Community

Derive The Relationship Between Half Life Period And Rate Constant Of A Zero Order Reaction
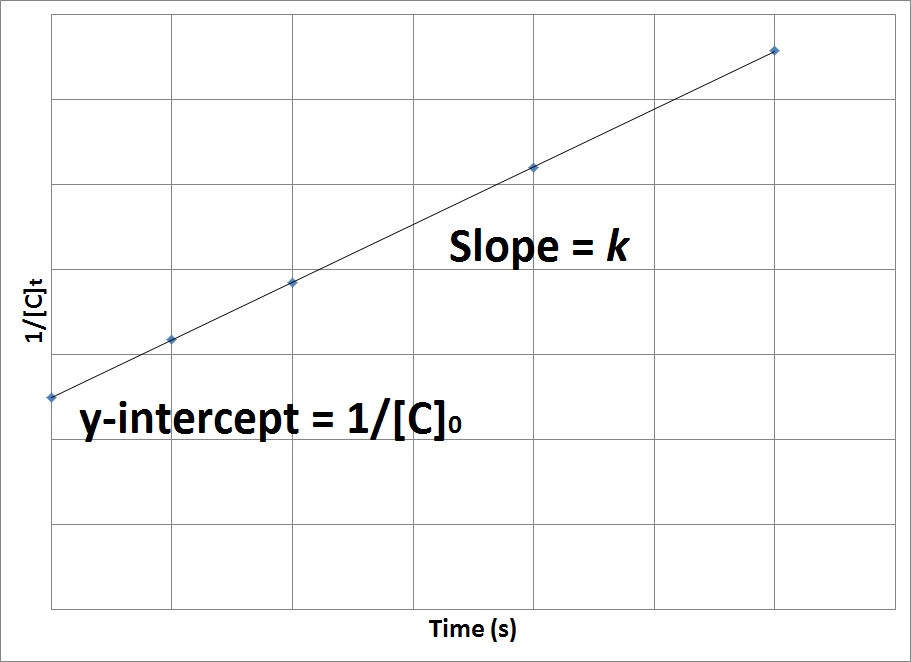
Concentration Time Relationships Integrated Rate Laws Introductory Chemistry 1st Canadian Edition

A Is Independent Of The Initial Concentration Of The Reactant

Integrated Rate Laws Chemistry For Majors